What is Corrosion?
Corrosion is a naturally occurring process of materials returning to their natural state. As with any man-made material, a considerable amount of energy is put into the finished product through mining, smelting, and processing. It’s the act of corrosion that reverts these finished products to their original form.
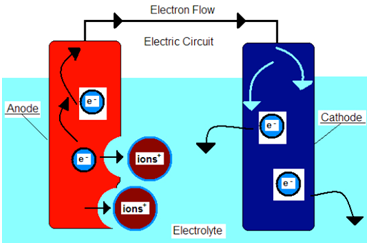

Above left, we see the corrosion cell. It is made up of 4 components: An Anode, a Cathode, an Electrolyte, and an Electrical Conductor to complete the circuit. On the right, we can see the Galvanic series which ranks metals from most to least noble, or more Cathodic to More Anodic. Both are important to understand why corrosion occurs on vessels and how best to prevent it.
Why is Corrosion a Problem for Vessels?
Let’s consider steel, represented as the Cathode in the above diagram.
In marine applications, steel – such as a ship’s hull plating-will want to decompose down to its fundamental elements (iron, carbon, oxygen, etc.) This happens because the positive ions in the steel (formed from the added energy during manufacturing)are released into the seawater during this breakdown into its natural form. This is commonly called rust. This process is further accelerated by the submersion into seawater, represented as the Electrolyte in the above diagram.
All underwater steel components in a vessel including the hull, rudder, bilge keels, etc. are constantly being bombarded with constant active corrosive unless they are properly protected.
Corrosion Prevention for Your Vessel – What are your options?
Since it’s a natural process, we can never fully stop corrosion from happening. We can, however, try to protect the Steel from degradation. This is achieved by applying these corrosion protection methods:
Sacrificial Anodes – “They give so your ship lives”
If you look at the above diagram on the right, we see that Steel is ranked nobler than Zinc. This means that if both metals are attached to the same corrosion cell, then Zinc would become the anode and Steel would become the cathode.

This results in the positive ions from the steel being released into the seawater like normal, however, instead those ions are now being replaced from the positive ions from the Zinc, thus the Steel cathode remains un-affected from corrosion.
This is why sacrificial anodes are commonly referred to as ‘Zincs’. Sacrificial anodes – they give so your ship lives!
Impressed Current Cathodic Protection
An alternative option for cathodic protection is the installation of an ICCP system. While its framework is slightly more complex, its functionality remains the same.
Instead of using lesser noble metals like Zinc to protect the Steel, ICCP systems use more noble metals like Titanium and/or Silver alloys.
These precious metals are formed into a cylindrical shape and attached to the ship in an out-of-the-way place, normally inside the sea chests.


This results in the Steel now becoming anodic to the Titanium; however, the lost ions are now supplied by an electric DC generator that is attached to the hull inside the vessel’s engine room. The electric current is regulated by multiple ICCP reference cells that monitor the underwater electric activity and provide the correct amount of output to adequately protect the Steel.
Coatings
The most basic form of corrosion protection is covering the hull with a protective coating. In theory, this will insulate the Steel from the seawater completely. These coatings are usually epoxy-based and are painted onto the vessel during construction and dry dock.
Whilst a lot of advances have been made in coating compositions over the years, these are easily removed from the hull due to marine growth and abrasions. This is why this form of protection is usually used in conjunction with either sacrificial anodes or an ICCP system.
In Summary
As each corrosion protection method has its advantages and disadvantages, it is important to choose the correct system for your vessel and keep it well maintained. Improper upkeep of these systems can leave your vessel vulnerable to immediate and aggressive corrosion causing serious consequences.
Professional Hull Cleaning and Underwater Ship Repair Company
Thai Subsea Services is the number one, all-encompassing commercial diving company for all types of Cathodic Protection systems in Thailand.
Contact us today to receive a free quotation. Get exclusive discounts upon registering for the entire fleet!


 The Importance of Proper Rudder Maintenance Practices
The Importance of Proper Rudder Maintenance Practices



